https://doi.org/10.1351/goldbook.A00381
In the representation of stereochemical relationships 'anti' means 'on opposite sides' of a reference plane, in contrast to 'syn' which means 'on the same side', as in the following examples.
- Two substituents attached to atoms joined by a single bond are anti if the torsion angle (dihedral angle) between the bonds to the substituents is greater than $\pu{90\!^{\circ}}$, or syn if it is less than $\pu{90\!^{\circ}}$. (A further distinction is made between antiperiplanar, synperiplanar, anticlinal and synclinal.)
- In the older literature the terms anti and syn were used to designate stereoisomers of oximes and related compounds. That usage was superseded by the terms 'trans' and 'cis' or E and Z, respectively.
- When the terms are used in the context of chemical reactions or transformations, they designate the relative orientation of substituents in the substrate or product:
- Addition to a carbon-carbon double bond:
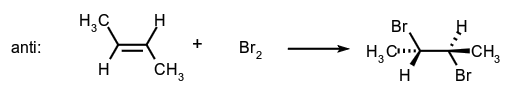
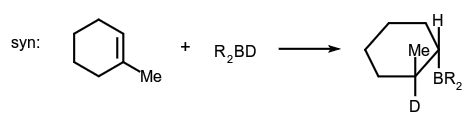
- Alkene-forming elimination:
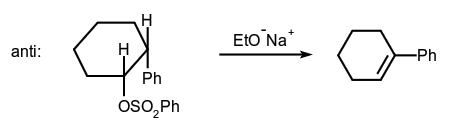
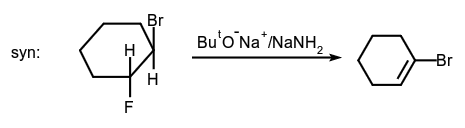
- Addition to a carbon-carbon double bond:
See also: endo, exo, syn, anti