https://doi.org/10.1351/goldbook.09165
Titration in which the electric conductivity of a solution is measured as a function of the amount of titrant added.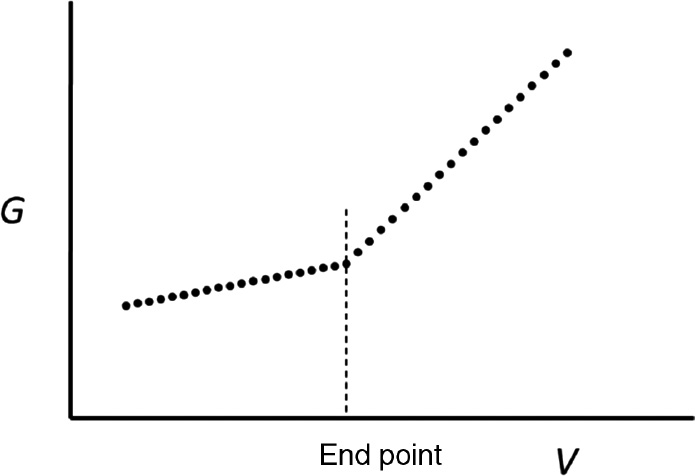
Notes:
- The method is based on replacing an ionic species of the analyte with another species, corresponding to the titrant or the product with significantly different conductance.
- The equivalence-point is obtained as the intersection of linear parts of the conductance \(G\), versus titrant volume \(V\), curve (see figure).
- The method can be used for deeply coloured or turbid solutions. Acid-base and precipitation reactions are most frequently used.

Conductimetric titration curve of conductance ($G$) against volume of titrant. The end-point of the titration is indicated by a change in slope of the graph.
Source:
PAC, 2020, 92, 641. 'Terminology of Electrochemical Methods of Analysis (IUPAC Recommendations 2019)' on page 685 (https://doi.org/10.1515/pac-2018-0109)
PAC, 2020, 92, 641. 'Terminology of Electrochemical Methods of Analysis (IUPAC Recommendations 2019)' on page 685 (https://doi.org/10.1515/pac-2018-0109)