https://doi.org/10.1351/goldbook.08190
Compound with unpaired electrons for which no Lewis structures are possible with all bonding electrons paired in single or double bonds.
Example: 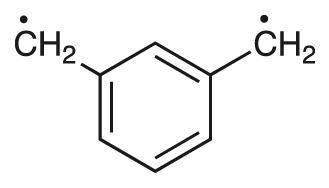
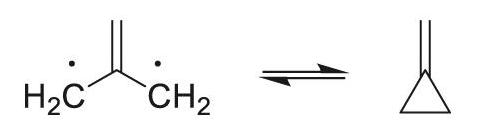

Note: The isomer shown on the right (valence tautomer methylidenecyclopropane) is a Kekulé structure.
Source:
PAC, 2022, 94, 353. 'Glossary of terms used in physical organic chemistry (IUPAC Recommendations 2021)' on page 472 (https://doi.org/10.1515/pac-2018-1010)
PAC, 2022, 94, 353. 'Glossary of terms used in physical organic chemistry (IUPAC Recommendations 2021)' on page 472 (https://doi.org/10.1515/pac-2018-1010)